7th World Congress for Hair Research (2013)
Collapse
X
-
There's a billion reasons why a company would want to do R&D in the US I think the most relevant here is knowing that whatever they come up with they can patent in a country where the rule of law is actually enforced, not to mention the fact that if you hire someone and then want to fire them there is no BS laws or constitutional clause making you potentially liable for a lawsuit (think France, Italy, Spain, Greece, etc. - no offense to our European friends...).
Ultimately the US is still the most business friendly place on earth, with maybe a few exceptions like countries like Singapore which are stil obviously not the potential market the US can be.Leave a comment:
-
-
why the hell did aderans chose USA and the FDA to run through with their clinical trials? All its done is give them a shitload of hoops to jump through.I'm not so sure they would have to start from scratch again. If you read the Replicel thread you will see that they have changed their cell culturing protocol from phase I but they're still moving into phase II not doing a new phase I. Maybe Aderans would have to do phase II again with the new spherical culturing but it'd be a much shorter phase II cos they hopefully now know which is the best version of Ji Gami. I dunno I'm sure there'll be some reason or other why it'll take years but if Replicel can change culturing protocol without starting over I'm sure Aderans should be able too. Unless it's cos FDA are stricter than the Germans.Leave a comment:
-
I'm not so sure they would have to start from scratch again. If you read the Replicel thread you will see that they have changed their cell culturing protocol from phase I but they're still moving into phase II not doing a new phase I. Maybe Aderans would have to do phase II again with the new spherical culturing but it'd be a much shorter phase II cos they hopefully now know which is the best version of Ji Gami. I dunno I'm sure there'll be some reason or other why it'll take years but if Replicel can change culturing protocol without starting over I'm sure Aderans should be able too. Unless it's cos FDA are stricter than the Germans.Leave a comment:
-
I was thinking this too. If they released their current method as a treatment now, I'd definitely go for it, and go for another treatment with their update.Even with your own cells, there can be issues with implanting them. Particularly if they are stem cells, as they are totipotent (able to differentiate into any types of cell) or pluripotent (able to differentiate into many types of cells). Given that they have this totipotency, f they receive the wrong "messages" (as it were) from the body, then there is a very large breadth of tissue types they could develop into. Ones which you would obviously not want in your scalp.
There could also be an issue with stem cells that refuse to die - think cancer.
So there is a high chance they would have to start again. Or maybe start running this trial parallel to their current one, and almost release it as an "update" in the future. Hair v1.1.Leave a comment:
-
Even with your own cells, there can be issues with implanting them. Particularly if they are stem cells, as they are totipotent (able to differentiate into any type of cell) or pluripotent (able to differentiate into many types of cells). Given that they have this totipotency, f they receive the wrong "messages" (as it were) from the body, then there is a very large breadth of tissue types they could develop into. Ones which you would obviously not want in your scalp.
There could also be an issue with stem cells that refuse to die - think cancer.
So there is a high chance they would have to start again. Or maybe start running this trial parallel to their current one, and almost release it as an "update" in the future. Hair v1.1.Leave a comment:
-
-
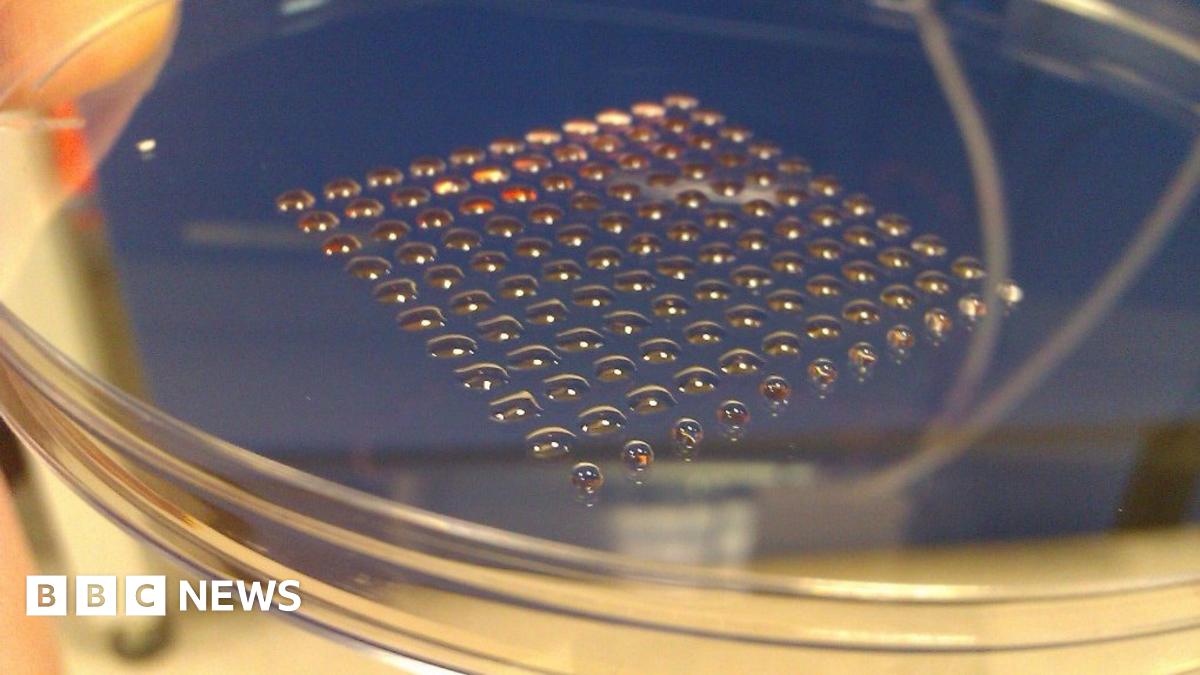
dr nigam doesn't need to hold safety trials. he could use this spheroid technique to mass produce dp cells. anything else would require at least 10 years.This is all great Desmond but how long will it realistically be before anything comes of this? It all seems so easy, Jahoda just needs to speak to the Scots, Aderans needs to speak to Jahoda and then Aderans should use this spheroid culturing technique in the phase III trials. The meeting this week is the perfect opportunity for these guys to get together, I wish that they use it to everyone's advantage and don't just waste it.Leave a comment:
-
lol one day a scientist will read the Desmond thread and they will learn from him and then do it and find the cureLeave a comment:
-
Leave a comment:
-
I'm curious about this actually! Can they just substitute this with their current method without having to start Phase I and II all over again?This is all great Desmond but how long will it realistically be before anything comes of this? It all seems so easy, Jahoda just needs to speak to the Scots, Aderans needs to speak to Jahoda and then Aderans should use this spheroid culturing technique in the phase III trials. The meeting this week is the perfect opportunity for these guys to get together, I wish that they use it to everyone's advantage and don't just waste it.Leave a comment:
-
This is all great Desmond but how long will it realistically be before anything comes of this? It all seems so easy, Jahoda just needs to speak to the Scots, Aderans needs to speak to Jahoda and then Aderans should use this spheroid culturing technique in the phase III trials. The meeting this week is the perfect opportunity for these guys to get together, I wish that they use it to everyone's advantage and don't just waste it.Leave a comment:
-
Here's the link to the article btw:
Leave a comment:

Leave a comment: