I've read many articles on a Japan based company called shisiedo who hope to release a new treatment, cure for MPB by 2020.. Has anyone else heard of this company and RCH-01 before? It seems extremely promising for basically anyone who suffers from hair loss regardless the aggressiveness and extent.
1. Shiseido/Replicel Ė Replicelís hair growth treatment, RCH-01, involves culturing a personís own hair follicle cells and then re-injecting them back into their scalp. First, a small punch-biopsy is removed from a personís healthy hair follicles. Then, a specific cell is dissected from the follicle and cultured in a growth medium. The cells are replicated into the millions and then injected back into the personís scalp. There is a short video about the procedure here. Replicel has been involved in a lot of activity over the past years to further develop their RCH-01 treatment into a worldwide success. For starters, Replicel created a partnership with Shiseido, which is the fourth largest cosmetic company in the world. In May 2014 Shiseido opened a huge biotechnology facility in Japan to accelerate the launch of Replicelís hair technology RCH-01. This endeavor coincides perfectly with Japanís new legislation which is designed to help expedite the trial process for stem cell technologies. In other words, Japan is an ideal place to launch a new cellular-based technology and get it to market quickly. To be clear, Shiseido and Replicel are separate companies. Replicel has licensed Shiseido the rights to bring RCH-01 to the world through an expedited regulatory path in Japan. It is worth mentioning that Shiseido also has its own scientists who are working on a separate hair regeneration technique using iPS cells. A treatment using iPS cells would be very advanced and could potentially produce an unlimited amount of hair regrowth.
Points of Interest: Shiseido is currently trialing Replicelís RCH-01 in Japan for market approval by the end of 2018. Replicel is also planning on launching its own RCH-01 Phase 2 trial, hopefully sometime in 2017, for the regulatory approval pathway in North America.
1. Shiseido/Replicel Ė Replicelís hair growth treatment, RCH-01, involves culturing a personís own hair follicle cells and then re-injecting them back into their scalp. First, a small punch-biopsy is removed from a personís healthy hair follicles. Then, a specific cell is dissected from the follicle and cultured in a growth medium. The cells are replicated into the millions and then injected back into the personís scalp. There is a short video about the procedure here. Replicel has been involved in a lot of activity over the past years to further develop their RCH-01 treatment into a worldwide success. For starters, Replicel created a partnership with Shiseido, which is the fourth largest cosmetic company in the world. In May 2014 Shiseido opened a huge biotechnology facility in Japan to accelerate the launch of Replicelís hair technology RCH-01. This endeavor coincides perfectly with Japanís new legislation which is designed to help expedite the trial process for stem cell technologies. In other words, Japan is an ideal place to launch a new cellular-based technology and get it to market quickly. To be clear, Shiseido and Replicel are separate companies. Replicel has licensed Shiseido the rights to bring RCH-01 to the world through an expedited regulatory path in Japan. It is worth mentioning that Shiseido also has its own scientists who are working on a separate hair regeneration technique using iPS cells. A treatment using iPS cells would be very advanced and could potentially produce an unlimited amount of hair regrowth.
Points of Interest: Shiseido is currently trialing Replicelís RCH-01 in Japan for market approval by the end of 2018. Replicel is also planning on launching its own RCH-01 Phase 2 trial, hopefully sometime in 2017, for the regulatory approval pathway in North America.
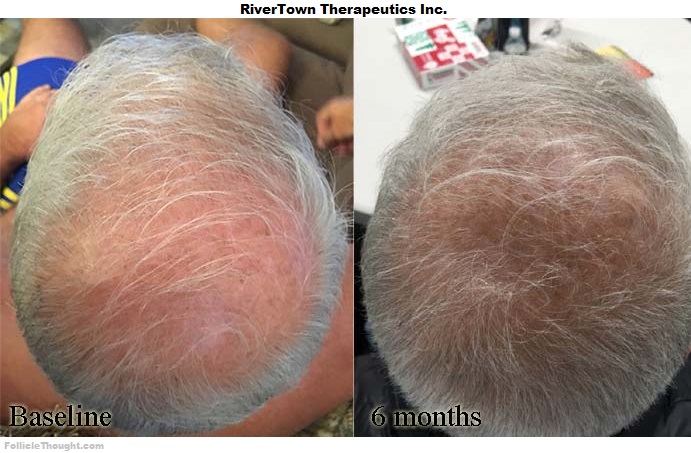
Comment