Both sodium valproate and VPA are very soluble in vehicles with 30% (or more) ethanol. The human AGA study I think used 27% ethanol with sodium valproate. To have the concentration you want (around 7% or 8%) you probably need the ethanol there or it might separate. I've made a lot of mixtures with VPA and various other components, and the ethanol seems necessary to have useful concentrations.
Updated Research and Knowledge - Cutting Edge
Collapse
X
-
Bej,
I have a very very importante question ( It's Seuxin...form HairLossHelp too ).
).
About VPA, what is important....the amount of VPA per day, or the % ?
By example :
If i use VPA at 7% in stemoxydine, and if i use 6ml per day of stemox...it represent more VPA ( as mg) than if i use 1-2ml of solution, right ?
For RU, CB, or Minox for example the important is the amount of solution...but i don't know if for acidic solution it's the same.
Do you understand my question ? What do you think ?
ThanksComment
-
If you're okay with castration then yes. It will go systemic even if you use it as topical. You want a special AR blocker that doesnt function in the blood stream. Like CB or RU, or a combination of the experimental ones in this thread.
Here's a good place to start:Chemical,
I've followed this thread with interest and appreciate all your hard work and research. I'd like to replicate your treatment on myself, but I don't have a chemistry background and want to make sure I'm using the right products and dosages. Is it possible to go into the details of your current regimen so that the followers of this thread can easily replicate what you're doing? If you can provide exact dosages and even brand names you've been using to buy some of these products, that would be really helpful. Thanks again!
Summary - First Post The original thread (https://www.baldtruthtalk.com/threads/22104-Updated-Research-and-Knowledge-Cutting-Edge/) was becoming too big and it was becoming difficult for users to find the more important information which prompted the need for a new thread will all the research and protocols condensed into a
My protocol is always changing so you're better off sticking to the main components:
Ketoconazole cream 2% - Daktarin - 2x day
Kirkland minox 5% - 2x day
Borage oil - Solgar (capsules poured into dispenser) - night
Evening primrose oil - Natures Aid capsules (same dispenser) - night
Oleuropein - Swanson (1mg/ml in minox) - night (stopped for now)
Sodium Valproate tablets crushed - 10mg/ml in distilled water and equal amount of minox solution (stopped for now)
Teavigo EGCG - Swanson - 8-10/mg/ml in separate minox solution - night (stopped)
Hey machi, I want to tell you that I know what it feels like but I havent had it for that long so I can only imagine how hard it is for guys like you. Growing hair is a difficult process and it takes alot time, for some people nothing seems to work and it can be frustrating so I'd encourage you try and stay hopeful. We're in the 21st century and there are alot of promising treatments on the horizon. I'm not going to give you false hope by saying the research we're doing here is going to lead to a cure but we've made some huge leaps in understanding AGA better which will increase the chances of coming across something that helps. You can try the treatments listed in this thread but it will take time and perseverance to see results especially given that you've had AGA for a while. Stay strong.chemical , I have no knowledge of alopecia. I suffer aga for 16 years . I was deceived by a surgeon in Spain twice and had to go to Dr. Hasson to solve the problem .
I took finasteride for 7 years with very good results but I gave it up because of side effects . 8 years ago I left finasteride.
currently I do not take drugs for hair loss and I 'm sentenced because I have several capillaries operations.
I read your posts but I do not understand anything . I'm depressed , bitter. please chemical , you seem to have much knowledge. I think we are many users who believe in you. Do you think you come to any conclusion on your research and get a possible future treatment ?
I like the authors scientific approach but he seems to use alot of associative reasoning. It pains me to see scientific minds become a victim of confirmational bias, they end up looking for literature that reaffirms their hypothesis and form conclusions based on loose inductive logic.Chemical, have you seen:
which if correct indicates that Estrogen plays a negative role (causes terminal hair - vellus, is involved in fibrosis of scalp around follicles, etc) rather than a positive role?
He believes AGA is more a lifestyle/endocrinology related pathogenesis. Oxidative stress, Immune system, prostaglandins, glucose metabolism, diet, exercise - yeah they all play a part just like breathing provides oxygen to all mitochondria in all the cells. Side effects and symptoms of AGA do not mean that they are causative factors. Alot of people are in the PGD2 bandwagon and with good reason, but InBeforeTheCure has demonstrated that this whole PGD2 thing is caused by Androgens. How are you going to deny that Androgens arent the main factor driving the progression of baldness?The heavy focus on androgens and “the genes” as causes of baldness have led people to believe that pattern hair loss is a compartmentalized problem rooted in vanity that has nothing to do with their metabolism or lifestyle. Confusion about the role of androgens probably relates to testosterone’s conversion into estrogen during metabolic stress and that dihydrotestosterone (DHT), like DHEA, can increase to buffer the effects of metabolic stress, for example, as an anti-estrogen.
There is fibrosis in AGA. Estrogens, prostaglandins and histamine are involved in the development of fibrosis. Therefore, Estrogen is bad. Also AGA has three letters. A triangle has three sides. The illuminati created AGA.Estrogen, free fatty acids, prostaglandins, mast cells, and histamine are involved in the development of fibrosis, or the abnormal progression of the normal formation of fibrous material between cells due to inflammation. In 1992, Jaworsky et al. found that a prominent feature of baldness was mast cell degranulation and the activation of fibroblasts resulting in fibrotic thickening of the hair follicle.[28] In another experiment, 412 people with pattern baldness (193 men and 219 women) confirmed the presence of a significant degree of perifollicular fibrosis in at least 37% of cases. Moreover, balding men with higher levels of inflammation and fibrosis led to worse outcomes using the traditional hair loss remedy minoxidil compared to those with lower levels.[29]
I've had this debate about fibrosis and AGA in the past, feel free to read the exchange with youngin from this post onwards. In short, the Reactive oxygen species generated by the DPC as a result of Androgen overexpression leads to excessive TGF-beta production. TGF-beta has been shown to kick off the chain that causes fibroblasts to deposit fibrotic tissue.
Estrogens are actually beneficial to frontal hair follicles, being able to stimulate the elongation of hair shafts - I made an in-depth post here here.
Correct, both VPA and SV are soluble in most solvents and I highly doubt you've want to go beyond 30mg/ml anyway. The only reason I suggested water is because its easier for people to make a solution without too much effort. I have a strong suspicion the study used ethanol to enhance the skin penetration seeing as ethanol strips the lipid barrier and weakens the stratum corneum. I personally encourage the use of ethanol + PG as a primary vehicle for nearly everything. Ideally a you'd want a water based solution with any amount of ethanol (maybe PG too) to enhance skin permeation but I dont see why a water based solution wouldn't work in this case for people who cant get hold of 190 proof alcohol.Both sodium valproate and VPA are very soluble in vehicles with 30% (or more) ethanol. The human AGA study I think used 27% ethanol with sodium valproate. To have the concentration you want (around 7% or 8%) you probably need the ethanol there or it might separate. I've made a lot of mixtures with VPA and various other components, and the ethanol seems necessary to have useful concentrations.
Regarding separation? - I've never seen a dissolved molecule separate from a solution before its reached saturation. But I'm not a molecular biologist and only have experience mixing water and sugar so correct me if I'm wrong lol.
and only have experience mixing water and sugar so correct me if I'm wrong lol.
Comment
-
@Chemical
Putting aside the negative effects and looking purely at the effects on hair, would Propecia and Nizoral 1% plus Minoxidil be enough to cover the Androgen angle to this or should I just use dut?Comment
-
ketoconazole cream 2% twice a day + finasteride + minox will cover the Androgen side if you've got good density or less aggressive AGA.
The problem with 5ar inhibitors, especially oral ones, is that they have a tendency to increase LH production and subsequently raise blood T levels and a local reduction in T's conversion to DHT leading to even more more T within cells. Suraphysical levels of T can activate the AR just as potently as DHT so it just offsets the potential gain from DHT inhibition. It's a tricky decision, yes Dut will kill more DHT, but you'll have even more T in comparison to Fin. I would stick to Fin.
Did you get the depakote ones or the epilium? It looks like the tablets have some coating, I want tablets without a coating, preferably capsules.
I think I'll scrap the whole mg/ml crap, so long as we know what doses work and there is okayish solubility we can just control the dose with the application. Go with 50/30/20 Ethanol/Water/PG and use as much mg as you want. You dont want the ethanol to dry up quickly. Given that the study used 8mg per application and some individuals probably used less or more, with some cases of slight systemic absorption I think it's pretty safe to bump the dose higher than 8mg. The plasma therapeutic range is 50-125 µg/mL according to google and the topical absorption didnt get anywhere near those numbers. The cases of hairloss associated with oral VA require chronic high doses and even then it only affects a small minority:
I believe the AR inhibitory properties are due to it's ER potentiating effects and I'm seeing more studies showing various ER agonists have the ability to downregulate AR in a dose dependent manner. The hairloss effects of VA could be more noticeable in women than men since female follicles require certain levels of ER to grow and maintain anagen. The study I posted before showed the AR suppressive effects were maximal at 2mM+ so I'm going to try and figure how that translates to topical use. I'm tempted to try alfatradiol (17-alpha Estradiol - available from Amazon.de as Ell Cranell) which supposedly increases the levels of Aromatase - more E2 should help VA work at a lower concentration. 17alpha Estradiol doesnt have the feminizing effects of 17beta Estradiol so you dont need to worry about gyno as long as the dose is low.http://www.bioline.org.br/pdf?pt05030
Among 211 patients who received sodium valproate (enteric coated tablet), 51% were male and 49% were female (no significant difference). Patients’ mean age of epilepsy onset was 11 years old and mean age starting to receive sodium valproate was 18 years old. More than 78% of patients had experienced tonic–clonic seizures (primary or secondary). All patients received monotherapy (single drug treatment). Overall, there were 6 cases (3.5%) of hair loss and curling of hair. Among them 3 cases were female and 3 were male. This side effect could be observed 3 months after first dose of therapy It took 1-2 years before appearance of hair loss and curly hair in other cases.
The powder dried up in the capsules and I need to buy more. I'm experimenting without it for now to see if I can get regrowth using other stuff.Comment
-
30 mg/mL (a.k.a. 3%) VPA or SV probably isn't going to be strong enough, considering that even at it's best, VPA/SV isn't a super robust hair grower. There was a mouse study that I think the human study used as a preliminary research. In mice, they tried several different concentrations of SV. 3% was not very effective as compared to around 7%.Correct, both VPA and SV are soluble in most solvents and I highly doubt you've want to go beyond 30mg/ml anyway. The only reason I suggested water is because its easier for people to make a solution without too much effort. I have a strong suspicion the study used ethanol to enhance the skin penetration seeing as ethanol strips the lipid barrier and weakens the stratum corneum. I personally encourage the use of ethanol + PG as a primary vehicle for nearly everything. Ideally a you'd want a water based solution with any amount of ethanol (maybe PG too) to enhance skin permeation but I dont see why a water based solution wouldn't work in this case for people who cant get hold of 190 proof alcohol.
Regarding separation? - I've never seen a dissolved molecule separate from a solution before its reached saturation. But I'm not a molecular biologist and only have experience mixing water and sugar so correct me if I'm wrong lol.
and only have experience mixing water and sugar so correct me if I'm wrong lol.
I believe the SV studies had to use ethanol simply for solubility reasons. Take a look at the molecule, it is, for the most part, a very fatty-like molecule. I've been making different formulations, and one batch I made had too much water & glycerin, and not enough ethanol & butylene glycol. The VPA completely separated. Then I remade this formula, but tweaked it to have less water/glycerin and more ethanol/BG, and the VPA was totally soluble. I took notes on all the amounts used, but I'm going from memory at the moment. If you look at the molecule though, it's obvious it can't dissolve in pure water.
Aha, here it is: a pubchem (good site to look up molecules) reference for VPA (it didn't have much to say about SV solubility). VPA in pure water will only go to about 1 mg/mL, or 0.1%. Although, you can't always trust these solubility numbers, sometimes it's less or more. But that sounds about right to me, because it's a mostly fat-like molecule.
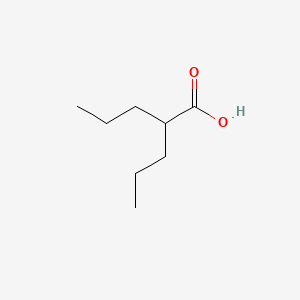
Valproic Acid | C8H16O2 | CID 3121 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
Ethanol isn't hard to get. You don't even need 95% Everclear. Most states that make it tough to get the 95% will offer 75.5% ethanol at the liquor store. And from 75.5%, you can easily add water to get down to ~30% ethanol.
I wouldn't recommend PG as a solvent, but rather BG instead. Butylene glycol has similar solvent properties to PG, but BG is typically a lot less irritating. For those buying all kinds of ingredients to use, it would be a shame to go cheap and have skin irritation with PG. I have to avoid PG or my scalp will break out, and a surprising number of people have PG sensitivity. I got BG from lotioncrafters online.
Another good solvent that is under-utilized by the hair loss community is glycerin. Glycerin also looks chemically a lot like PG, but that one extra -OH group makes a big difference. Rather than being an irritant, glycerin is a nutrient that moisturizes the skin, adds some viscosity to the formula (think of hand sanitizers), and the glycerin is actually a 3-carbon sugar that can be used as fuel by the skin cells, or other aspects of cell metabolism. Glycerin is good for skin at up to 20% in a formula.Comment
-
I couldn't find any images of the EC500 and was hoping to get them myself. If theres a coloured coating you might be able to dissolve it until you see the actual powder idk. You could try scraping it off too. I get mine from 4nrx (dirt cheap) and the orange coating just chips off with a little effort. If you cant get it off then it shouldn't cause too much of an issue, probably just sugar and colouring.
You're right I dont know why I keep thinking 1mg/ml = 1%. The study used 80mg/ml to get 8%. Also can you post the mouse study, I prefer not to go assumptions and random numbers. As for the dose, I've come to realise it's a bit useless working with the solubility if you can control how much of the actual drug you apply. If you went with ethanol and saturated completely to get 30mg/ml, you'd want 2ml to get 60mg and so forth. The human study advised subjects to use around 0.8ml/64mg:30 mg/mL (a.k.a. 3%) VPA or SV probably isn't going to be strong enough, considering that even at it's best, VPA/SV isn't a super robust hair grower. There was a mouse study that I think the human study used as a preliminary research. In mice, they tried several different concentrations of SV. 3% was not very effective as compared to around 7%.
The subjects were instructed to use either the VPA (sodium valproate, 8.3%) or placebo spray twice daily, with seven or eight pumps (~0.8 mL) for each dose.You might have missed this comment I made along with the source for the solubility numbersI've been making different formulations, and one batch I made had too much water & glycerin, and not enough ethanol & butylene glycol. The VPA completely separated. Then I remade this formula, but tweaked it to have less water/glycerin and more ethanol/BG, and the VPA was totally soluble. I took notes on all the amounts used, but I'm going from memory at the moment. If you look at the molecule though, it's obvious it can't dissolve in pure water.
Aha, here it is: a pubchem (good site to look up molecules) reference for VPA (it didn't have much to say about SV solubility). VPA in pure water will only go to about 1 mg/mL, or 0.1%. Although, you can't always trust these solubility numbers, sometimes it's less or more. But that sounds about right to me, because it's a mostly fat-like molecule.
Also pubchem didnt have anything on sodium valproate and the human study used sodium valproate. I dont think its fair to compare VPA and SV hence I looked deeperSince Sodium Valproate is the more readily available form and has excellent water solubility at 50mg/ml, in comparison to 1.3mg/ml for Valproic Acid (source) it looks like Sodium Valproate is a strong candidate to be used in our protocols.
 Valproic acid sodium salt, CAS: 1069-66-5, is an HDAC inhibitor with anticancer, anti-inflammatory and neuroprotective effects. Cited in 7 publications
Valproic acid sodium salt, CAS: 1069-66-5, is an HDAC inhibitor with anticancer, anti-inflammatory and neuroprotective effects. Cited in 7 publications
I trust scbt if pubchem doesnt list the exact molecule and both the pdf and scbt show the Sodium Valproate has 50mg/ml solubility in water. Whereas Valproic Acid specifically has very low water solubility corroborated by multiple sources and the trustworthy pubchem. You've also come to realise this with your own experiments that Valproic Acid isn't very poorly soluble in water. But the real question is did you try Sodium Valproate? I'm sure you acknowledge that the two are chemically different.
Good point but water is still an option. Not everyone is in the states remember!
The PG is mainly to prevent the ethanol from drying up too quick and give the molecules enough time to soak in after the barrier is weakened. As you can tell I dont know jack about chemistry so couldn't recommend anything other than PG even though many people are sensitive to it. Looks you are right about BG and glycerols being equally effective substitutes to PG - according to these guys. Glad we've finally got someone with a chemistry background to correct my mistakes!I wouldn't recommend PG as a solvent, but rather BG instead. Butylene glycol has similar solvent properties to PG, but BG is typically a lot less irritating. For those buying all kinds of ingredients to use, it would be a shame to go cheap and have skin irritation with PG. I have to avoid PG or my scalp will break out, and a surprising number of people have PG sensitivity. I got BG from lotioncrafters online.
Another good solvent that is under-utilized by the hair loss community is glycerin. Glycerin also looks chemically a lot like PG, but that one extra -OH group makes a big difference. Rather than being an irritant, glycerin is a nutrient that moisturizes the skin, adds some viscosity to the formula (think of hand sanitizers), and the glycerin is actually a 3-carbon sugar that can be used as fuel by the skin cells, or other aspects of cell metabolism. Glycerin is good for skin at up to 20% in a formula.Comment
-
Hi Chemical, thanks so much for all your time and effort. Can you go more into what you are saying about oral 5ar inhibitors please?ketoconazole cream 2% twice a day + finasteride + minox will cover the Androgen side if you've got good density or less aggressive AGA.
The problem with 5ar inhibitors, especially oral ones, is that they have a tendency to increase LH production and subsequently raise blood T levels and a local reduction in T's conversion to DHT leading to even more more T within cells. Suraphysical levels of T can activate the AR just as potently as DHT so it just offsets the potential gain from DHT inhibition. It's a tricky decision, yes Dut will kill more DHT, but you'll have even more T in comparison to Fin. I would stick to Fin.
When fin stopped working for me after years I switched to dut and got a couple years more. Now i'm loosing ground on 1 mg / day dut and have even added 8% ru daily.
This has been really hard on me and I don't know what to do anymore... these drugs worked super good for me and now they don't.. what can I do Chemical? Please any advice would be so much appreciated.
thanksComment
-
Well, I could be wrong, I guess. I've been wrong about many things before, and I'll be wrong about many things in the future.
Speaking of genetics, I've been playing around with something called RegulomeDB today. You put in a SNP or list of SNPs, and it combs through high-throughput experimental data and shows you epigenetic factors that those SNPs could be affecting. I threw in all the SNPs in Table S4 from this study, but focused mostly on the X-chromosome location (EDA2R/AR, with a bunch of SNPs in linkage disequilibrium) and the Chr20 location (around a long non-coding RNA segment called LINC01432 between PAX1 and FOXA2, again with a bunch of SNPs in linkage disequilibrium). The interesting thing about these two is that having the risk alleles for both can act synergistically to give you a 7x risk rather than the 2.8x or whatever it is you would get if you multiplied the odd ratios.Originally posted by ChemicalMy theory is that the frontal DPC's sensitivity to sex steroids has been unintentionally conserved by men when selecting women with thick hairlines, perceiving them to be more youthful and attractive. And this regional difference in DPC response becomes the complete opposite in males, but since women might have given less preference to looks and more on dominance, the genes ended up being conserved.
So...The strongest hit in the EDA2R/AR region is from rs6152 on AR, which is just under 1.7kb downstream of the AR promoter: http://regulome.stanford.edu/snp/chrX/66765626
You can see that EZH2 is there, so maybe the SNP affects EZH2 binding affinity. Since EZH2 is a methyltransferase, maybe rs6152 = A is more highly methylated (and therefore transcription of AR repressed). It's in a good spot for that too. See Fig1a: Negative correlation between methylation and expression is enriched in extensive regions downstream of promoters.
For the Chr20 locus, the highest score was with rs6137444 (position chr20:21785638), the most upstream of the Chr20 SNPs associated with AGA: http://regulome.stanford.edu/snp/chr20/21785638
As you can see, it's around an AR binding site. It probably has nothing to do with FOXA2, since FOXA2 isn't expressed at all in hair follicles. It may affect PAX1, which is expressed in DP cells and which plays a role in pattern formation. Or maybe the culprit is the long non-coding RNA -- long non-coding RNA can have epigenetic roles. Of note:
So maybe androgen-dependent AR binding to this site slowly and permanently changes the epigenetic program of DPCs, which could be irreversible even with AR knockdown. Who the hell knows? It's fun to speculate.Noncoding transcription has even been shown to induce the formation of heterochromatin at the p15 tumor suppressor gene locus that persisted after noncoding transcription was turned off, suggesting that the transient expression of ncRNAs can have long-lasting heritable effects on gene expression (Yu et al. 2008).
It would be an interesting experiment if some researcher could see what happens when LINC01432 is overexpressed vs. underexpressed, what roles it may have, whether AR increases or decreases its expression in the presence or absence of DHT, and so on. And then maybe do the same with PAX1.
Of course, these may not actually be the functional variants, but it's fun to play around with this stuff even with limited data.
I just Googled "site-specific gene therapy", and it seems to be a topic of ongoing research.Originally posted by ChemicalEven if we did have gene therapy, to me it seems like it would be very difficult to localise it to just the epidermis of the frontal scalp. And the cost might not justify not getting a transplant instead either from healthy regions or cultured DPC if they get that far in the future.
But these are specialized adult stem cells, so they'll be programmed a particular way, right?Originally posted by ChemicalMy belief is that the DPC become conditioned during embryogenesis or at some point in the womb and that this is only specific to the DPC. It could very well be that the entire frontal scalp contains the AGA code, but that just seems unintuitive. The stem cells should have the same code regardless of the location (I tell myself). I'm just trying to be optimistic with my delusions.
Can you give some examples of people who stopped responding to RU? I can't explain it (if they're using real RU and it hasn't degraded).Originally posted by ChemicalOne thing I find strange is how it takes quite a while for the initial recession to become noticeable but happens quite fast when stopping treatments. I remember reading a study on Hic5/ara55 (Androgen co-activator) and how it starts to get upregulated after puberty. And also reports of Testosterone/DHT being able to upregulate the expression of AR. Perhaps continued activation of AR causes permanent upregulation or enhanced stability of AR. Its also peculiar that RU users stop responding after a while - I mean its not like theres a negative feedback loop here? What do you think?
Yes, I will say that I trust some sources more than others, but I won't get into that here.Originally posted by ChemicalLooking at CB and RU they seem like the realistic and most ideal AR antagonists currently on the market, they have virtually no systemic effects on the HPTA or GnRH release and only work peripherally. Its just the price and lack of reputable sources (I'm a little cautious of chinese manufacturers) that put me off.
The interesting thing is that in AGA males, beta-catenin binding to AR is more enriched with Wnt signaling alone than with DHT alone. They do use a small DHT concentration though, so I imagine that could change with higher concentrations. Nevertheless, it seems like beta-catenin is still able to bind TCF/LEF even when bound to AR (the luciferase activity is high in AGA40M after Wnt despite the AR/beta-catenin binding stain being pretty dark). However, AR could still maybe act as a co-transcription factor at Wnt target genes and screw things up.Originally posted by Chemical
Functional localization and competition between the androgen receptor and T-cell factor for nuclear beta-catenin: a means for inhibition of the Tcf signaling axis
Also AR is upregulated significantly so that might play a part in increasing the receptor saturation ceiling. It also doesnt make the conclusion robust given that non-AGA DPC have less AR. I want to know what would happen if AR was boosted to AGA levels in non AGA DPC.
We know that DHT/T that causes the binding of β-catenin to AR, and the study showed that without DHT, WNT3a did activate the TCF/LEF genes. Something that worries me is that maybe even an antagonist that binds to AR can recruit β-catenin. I'm thinking if we can prevent the binding of β-catenin to AR, we might not have to worry about Androgens at all. Something that fits into the pocket in place of β-catenin! What about SARMS and steroids? I know there are steroids that have significantly higher AR binding affinities that could easily saturate the receptors, and most of them have altered anabolic:androgenic ratios. Perhaps its the androgenic part that causes this negative response of AGA? Edit: looks like R1881 elicits the same response as T/DHT
By the way:
Acetylation of β-Catenin by p300 Regulates β-Catenin-Tcf4 Interaction
Lysine acetylation modulates the activities of nonhistone regulatory proteins and plays a critical role in the regulation of cellular gene transcription. In this study, we showed that the transcriptional coactivator p300 acetylated β-catenin at lysine 345, located in arm repeat 6, in vitro and in vivo. Acetylation of this residue increased the affinity of β-catenin for Tcf4, and the cellular Tcf4-bound pool of β-catenin was significantly enriched in acetylated form. We demonstrated that the acetyltransferase activity of p300 was required for efficient activation of transcription mediated by β-catenin/Tcf4 and that the cooperation between p300 and β-catenin was severely reduced by the K345R mutation, implying that acetylation of β-catenin plays a part in the coactivation of β-catenin by p300. Interestingly, acetylation of β-catenin had opposite, negative effects on the binding of β-catenin to the androgen receptor. Our data suggest that acetylation of β-catenin in the arm 6 domain regulates β-catenin transcriptional activity by differentially modulating its affinity for Tcf4 and the androgen receptor. Thus, our results describe a new mechanism by which p300 might regulate β-catenin transcriptional activity.The better we understand it, the better chance we have of being able to do that, I guess. We'll be better able to predict the outcome of manipulating certain things.Originally posted by ChemicalMost treatments themselves do not contain the growth factors, they merely stimulate the release indirectly and I'm wondering if all epidermal cells have the ability to release paracrine growth factors. Furthermore, there is only so much you can proliferate the HF shaft with exogenous growth factors, which explains why people only see vellus hairs getting longer. A functioning anagen DPC with an adequate blood supply thickens the diameter of the hair shaft significantly and continually releases growth factors in comparison the infrequent applications of treatments that may or may not reach target tissues. I'm thinking if theres another way to activate TCF/LEF genes without β-catenin, that would be even better than using indirect growth factors.
An interesting note about TGF-beta from the Tasseff paper I posted a few months ago:Originally posted by ChemicalI remember making a post about tgf-beta and dug this up:
So TGF-beta can potentiate AR via autocrine loop? the f***, it just keeps getting worse
Androgen receptor transactivity is potentiated by TGF-b1 through Smad3 but checked by its coactivator Hic-5/ARA55 in balding dermal papilla cells
(was behind paywall but thats not going to stop me now)
So hic5/ARA55 is already upregulated in AGA when it starts and that is known to enhance AR. Then AR -> ROS -> TGF-beta -> more hic5/ARA55 ¬ (TGF-beta -> smad3 -> AR)
So it looks like hic5/ARA55 is bad, but is keeping check on the additional AR effect of TGF-beta. A powerful or mild anti-oxidant is probably all we need to fix this part but I think we should both look into hic5/ara55 and other co-activators that could be making AGA worse over time.
Induction of transforming growth factor-beta 1 by androgen is mediated by reactive oxygen species in hair follicle dermal papilla cells
We investigated the possibility that inhibitory signaling genes may be in the DP enriched group identified as LFO cluster 2, but not well described by the static intracellular expression model. We expect such signaling genes to display an increased expression 14 to 16 days after morphogenesis, near the on-set of catagen and before the sharp decline in the expanding population (Figure 4). Using this criterion, we identified 88 expression signals (relating to 74 unique genes; see Supplementary File S4). We observed that these expression signals, on average, are consistent with population driven changes until near catagen on-set, where they begin to increase more than what was explained by static intracellular expression assumed in the 2-population model (Supplementary Figure S10). Of these genes, 50 were annotated as extracellular genes which yields and enrichment p-value of 7.46E-18, improved enrichment over cluster 2 with a p-value of 3.63E-8. For a full list of significant enrichment categories see Supplementary File S5. Interestingly, this relatively short list includes Tgfβ2, which is currently thought to be one of the signaling molecules produced in DP cells to initiate apoptosis in hair epithelial cells at catagen on-set [7].
Given the observed expression signal, membership in DP enriched cluster 2, high enrichment for extracellular genes and inclusion of Tgfβ2, this list may contain potential targets for molecules that communicate an inhibitory signal from the DP to proliferating hair epithelial cells, closing a negative feedback loop. Obviously further experiments will be required to test this hypothesis; however, it does provide a starting point for future validation of the conclusions drawn above and, perhaps, even those identified in the model of Al-Nuaimi et al. [10].Probably some of the DKK1 is by the ROS -> p53 -> DKK1 pathway you pointed out (there's a p53 binding site on the DKK1 promoter), but also that tail-off effect is really similar to prostate cancer graphs/stain patterns of Wnt target genes with increasing concentrations of DHT, and DKK1 is a direct Wnt target gene. Adding DHT could possibly translocate beta-catenin to the nucleus if it's bound to AR and can ride AR into the nucleus.Originally posted by ChemicalAnd they only used DHT which leads me to believe AR will increase ROS regardless of β-catenin being present (or maybe there is some β-catenin being released intracellularly). Maybe in AGA the binding of β-catenin to AR doesnt affect the transcription of AR genes but binds - just because it can and by the time it reaches the nucleus it cant activate TCF/LEF. I was incorrect about β-catenin making the DPC response worse, it just cant target its canonical genes, thats all.
We also know that DKK-1 is induced by reactive species, specifically JNK mediated. And looking back over the research it looks like the DPC themselves produce DKK-1:
But I dont understand why ROS is induced in AGA DPC when in PC it represses it. The androgen receptor represses transforming growth factor-beta signaling through interaction with Smad3.
So although there are quite a few similarities its not quite apples to apples which irritates me.Comment
-
I am mainly working with VPA since that was what I first obtained, and I've got this bottle of it and don't want to waste it. I got the VPA before thinking through VPA vs. SV issues. Maybe I'll someday replace the VPA with SV, but the formulas I'm working on are complicated, and once I get the bugs worked out with VPA, I'll probably stick with that.Also pubchem didnt have anything on sodium valproate and the human study used sodium valproate. I dont think its fair to compare VPA and SV hence I looked deeper
 Valproic acid sodium salt, CAS: 1069-66-5, is an HDAC inhibitor with anticancer, anti-inflammatory and neuroprotective effects. Cited in 7 publications
Valproic acid sodium salt, CAS: 1069-66-5, is an HDAC inhibitor with anticancer, anti-inflammatory and neuroprotective effects. Cited in 7 publications
I trust scbt if pubchem doesnt list the exact molecule and both the pdf and scbt show the Sodium Valproate has 50mg/ml solubility in water. Whereas Valproic Acid specifically has very low water solubility corroborated by multiple sources and the trustworthy pubchem. You've also come to realise this with your own experiments that Valproic Acid isn't very poorly soluble in water. But the real question is did you try Sodium Valproate? I'm sure you acknowledge that the two are chemically different.
Take those product sheets with a grain of salt. The pharmaceutical lab I work in happened to have a small amount of SV, which I discreetly used for a couple solubility tests. The product sheet you link to says SV goes to 3% in ethanol, but I put SV in ethanol at 8%, and it was very easily soluble. Another example, Lithium Chloride that I dissolved in ethanol was only half as soluble as the numbers reported.
In solution, VPA and SV will be chemically identical. Take a look at the two structures side-by-side. You'll see that the only difference is in one single spot where VPA has a hydrogen (H) and SV has sodium (Na). In solution, that H, and that Na, float away (dissociate). All the other bonds in the molecule are permanent (covalent) bonds, and so those atoms continue to stick together. The sodium weighs more than the hydrogen though, so when you take that into account, that's why 8.3% SV will be equivalent to 7.2% VPA, the same number of molecules of valproate per volume of liquid. Also, the H that dissociates from VPA is what makes it an acid. I had concerns about the acidity, but those theoretical concerns turned out to be a non-issue.Comment
-
I have used VPA. I had a major shed. Do you experience the same?I am mainly working with VPA since that was what I first obtained, and I've got this bottle of it and don't want to waste it. I got the VPA before thinking through VPA vs. SV issues. Maybe I'll someday replace the VPA with SV, but the formulas I'm working on are complicated, and once I get the bugs worked out with VPA, I'll probably stick with that.
Take those product sheets with a grain of salt. The pharmaceutical lab I work in happened to have a small amount of SV, which I discreetly used for a couple solubility tests. The product sheet you link to says SV goes to 3% in ethanol, but I put SV in ethanol at 8%, and it was very easily soluble. Another example, Lithium Chloride that I dissolved in ethanol was only half as soluble as the numbers reported.
In solution, VPA and SV will be chemically identical. Take a look at the two structures side-by-side. You'll see that the only difference is in one single spot where VPA has a hydrogen (H) and SV has sodium (Na). In solution, that H, and that Na, float away (dissociate). All the other bonds in the molecule are permanent (covalent) bonds, and so those atoms continue to stick together. The sodium weighs more than the hydrogen though, so when you take that into account, that's why 8.3% SV will be equivalent to 7.2% VPA, the same number of molecules of valproate per volume of liquid. Also, the H that dissociates from VPA is what makes it an acid. I had concerns about the acidity, but those theoretical concerns turned out to be a non-issue.Comment
-
Reponse desired
Thanks for the great write up first off!
Secondly is there anything I can do without fin/minox? wanting kids soon and had a very bad reaction to minox (not itching but skin ageing).
Many thanks,Comment

Comment